Chemical bonding: ionic bonds
Ions
Ions are produced when electrons transfer entirely from one atom to another. These are charged species. The charge of an ion depends on whether the atom gains (negatively charged or anions) or loses electrons (positively charged or cations).
Examples: \(\ce{Na}^{+}\), \(\ce{Ca}^{2+}\),\(\ce{Al}^{3+}\), \(\ce{Cl}^{-}\), \(\ce{O}^{2-}\), \(\ce{N}^{3-}\)
Octet Rule
The valence electron configuration of noble gases is considered the most stable valence electron configuration. Except for helium, all noble gases have eight electrons in their valence shell (completely filled \(s\) and \(p\) subshells). The main group metals attempt to achieve the electron configuration of the noble gas just before them in the periodic table by losing electrons. Main group nonmetals acquire the electron configuration of the noble gas just after them in the periodic table by gaining electrons.
For instance, potassium’s electron configuration is \(1s^{2}\,2s^{2}2p^{6}\,3s^{2}3p^{6}\,4s^{1}\). Potassium loses one electron (\(4s\) electron) and becomes potassium cation (\(\ce{K}^{+}\)) to achieve noble gas electron configuration: \(1s^{2}\,2s^{2}2p^{6}\,3s^{2}3p^{6}\).
The electron configuration of chlorine is \(1s^{2}\,2s^{2}2p^{6}\,3s^{2}3p^{5}\). To achieve noble gas electron configuration, chloride gains one electron (\(3p\) electron) and becomes chloride anion (\(\ce{Cl}^{-}\)) : \(1s^{2}\,2s^{2}2p^{6}\,3s^{2}3p^{6}\). Therefore, in general, metals form cations and nonmetals form anions.
When forming bonds, atoms attempt to achieve stable noble gas configuration by sharing, losing or gaining electrons for each of the atoms associated.
Naming Monoatomic Ions
Main group cations are named using the name of the element followed by ion. For instance, \(\ce{Li}^{+}\): Lithium ion, \(\ce{Mg}^{2+}\): Magnesium ion and \(\ce{Al}^{3+}\): Aluminium ion.
Transition metals (\(d,f\)blocks) and some \(p\) block elements can form multiple cations such as \(\ce{Cu}^{+}\) and \(\ce{Cu}^{2+}\), \(\ce{Pb}^{2+}\) and \(\ce{Pb}^{4+}\). These types of cations can be named in two ways. The first is that the ion with the smaller charge ends with “-ous” and the larger charge ends with “-ic”. For instance, \(\ce{Cu}^{+}\)is cuprous ion and \(\ce{Cu}^{2+}\) is cupric ion. The other is writing the charge of the ion in Roman numerals within parenthesis next to the element name. For example, \(\ce{Cu}^{+}\)is Copper\(\left(\mathrm{I}\right)\) ion, and \(\ce{Cu}^{2+}\) is Copper\(\left(\mathrm{II}\right)\).
Anions are named by removing the ending of the element name and replacing it with “-ide”. For instance, the anion formed by \(\ce{Cl}\) is the Chloride ion.
Polyatomic Ions
Polyatomic ions consist of more than one atom. For example, \(\ce{SO}_{4}^{2-}\)(sulphate), \(\ce{NO}_{3}^{-}\) (nitrate), \(\ce{PO}_{4}^{3-}\) (phosphate) and \(\ce{NH}_{4}^{+}\) (ammonium) ions. Atoms in polyatomic ions are held together by covalent bonds.
Isoelectronic Species
Isoelectronic species are atoms and ions that have an equal number of electrons. For example, \(\ce{O}^{2-}\), \(\ce{F}^{-}\), \(\ce{Ne}\), \(\ce{Na}^{+}\) and \(\ce{Mg^{2+}}\). All of these species contain \(10\) electrons.
Ionic Bonds
Ionic bonds are created between metals and non-metals by transferring electrons from metals to non-metals. Metals provide the cation (lose electrons), and nonmetals provide the anion (gain electrons). Generally, bonds formed between atoms with larger electronegativity 1 (e.g. elements in groups 16 and 17 of the periodic table) and smaller electronegativity (e.g. elements in groups 1 and 2 of the periodic table) are ionic. For instance, \(\ce{NaCl}\): difference between electronegativities of \(\ce{Na}\) (EN\(=0.93\)) and \(\ce{Cl}\) (EN\(=3.16\)) is \(2.23\). For an ionic compound to be formed, the difference between electronegativities of two participating atoms must be larger than or equal to two. The larger difference in electronegativity creates electrostatic interactions between the electrons of one atom and the nucleus of the other atom. 2
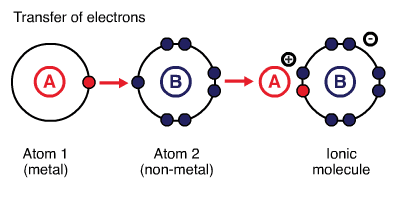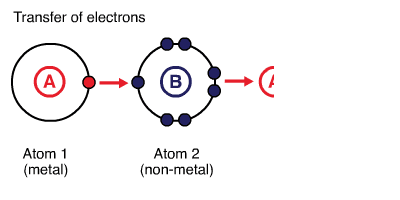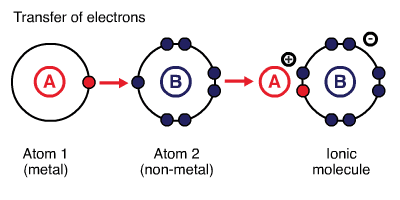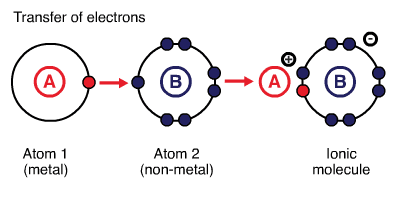
Ionic Compounds
Ionic compounds are held together by electrostatic interactions between cations and anions involved. The cations and anions are arranged in such a way that gaps between ions are filled efficiently and permit maximum electrostatic interactions. Ionic compounds are polar materials.
Writing Chemical Formulas for Ionic Compounds
Ionic compounds are neutral species. Ions involved in an ionic compound combine in such a ratio that the net charge becomes zero. Parentheses are used to enclosed polyatomic ions when more than one polyatomic ions are involved in an ionic compound. In that case, the subscript is written outside the parentheses. For instance, \(\ce{Fe}_{2}\left(\ce{SO}_{4}\right)_{3}\) and \(\left(\ce{NH}_{4}\right)_{2}\ce{CO}_{3}\). The chemical formula of an ionic compound displays the lowest possible ratio that ions can combine.
Naming Ionic Compounds
- First, write the name of the cation followed by the anion’s name with a space in between. For example, \(\ce{NaCl}\) is written as sodium chloride, \(\ce{MgSO4}\) is written as magnesium sulphate.
- Transitional metals and some of the \(p\) block elements have more than one charge. Therefore, if the cation is from those groups, it is essential to include the charge within parentheses next to its name in Roman numerals or use “-ous” and “-ic” to indicate smaller charge and larger charge, respectively. For instance, \(\ce{CuSO4}\) is written as copper\(\left(\mathrm{II}\right)\) sulphate or cupric sulphate, \(\ce{FeCl2}\) is written as iron\(\left(\mathrm{II}\right)\) chloride or ferrous chloride.
Physical Properties of Ionic Compounds
- Crystalline solids
- Conduct electricity in solution state
- High melting points and boiling points
- Soluble in polar solvents
1Electronegativity is the power of an atom in a molecule to attract electrons.
2The attraction forces between oppositely charged ions are known as electrostatic interactions.
