Strength of acids and bases
\[\require{mhchem}\]
Strong Acids and Weak Acids
Acids can be categorised as strong acids or weak acids, depending on their ability to completely transfer protons in an aqueous solution. Strong acids can transfer nearly all (100%) of their protons (hydrogen ions) to the water. Weak acids can only transfer some of their protons to the water.
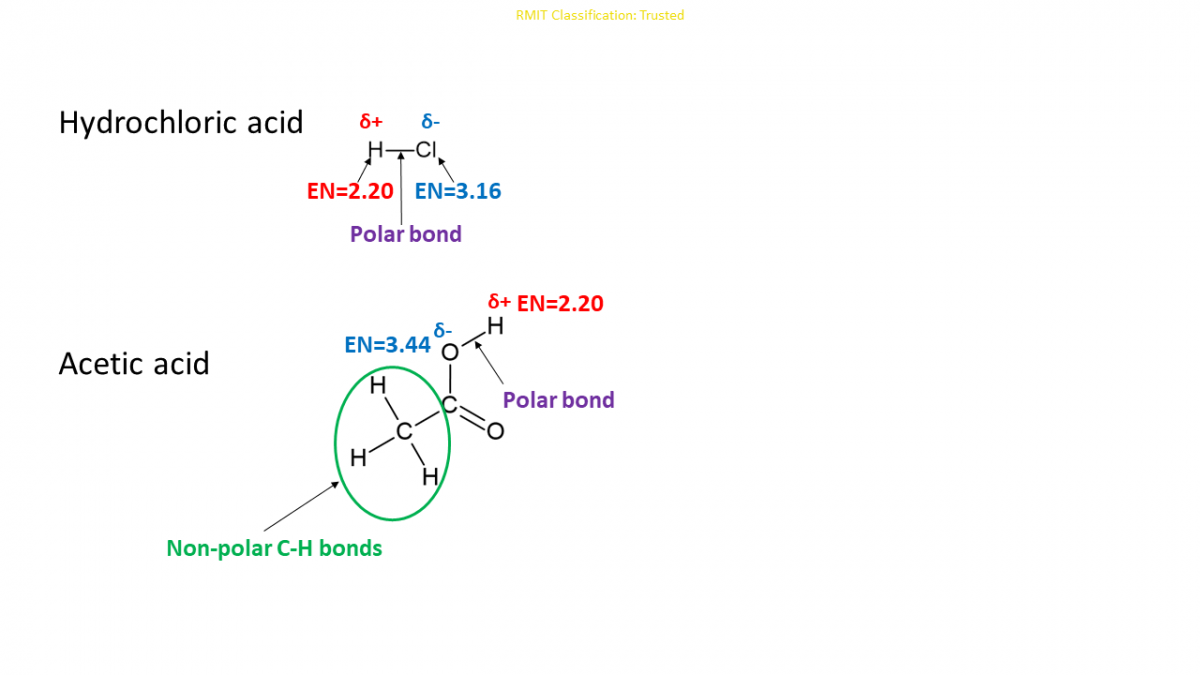
The extent of electron transfer depends on the molecular structure of acids. If the hydrogen is bound to a highly electronegative1 atom like oxygen or chlorine, that hydrogen is acidic and is able to undergo complete ionisation. For instance, hydrochloric acid (\(\ce{HCl}\)). If hydrogen is bound to a less electronegative atom like carbon, the bond is not polar enough to release hydrogen. For instance, hydrogen bound to carbon in acetic acid (\(\ce{CH}_{3}\ce{COOH}\)). Acetic acid has four hydrogens: one bound to oxygen and the other three to carbon. Only the hydrogen bound to oxygen is released as acidic hydrogen, making acetic acid a weak acid. \[\begin{align*} \ce{HCl}\left(g\right)+\ce{H}_{2}\ce{O}\left(l\right) & \rightarrow\ce{H}_{3}\ce{O}^{+}\left(aq\right)+\ce{Cl}^{-}\left(aq\right)\\ \ce{CH}_{3}\ce{COOH}\left(aq\right)+\ce{H}_{2}\ce{O}\left(l\right) & \rightarrow\ce{H}_{3}\ce{O}^{+}\left(aq\right)+\ce{CH}_{3}\ce{COO}^{-}\left(aq\right) \end{align*}\]
Most of the acids are weak acids. The common strong acids are listed below.
| Chemical formula | Name |
|---|---|
| \(\ce{HCl}\) | Hydrochloric acid |
| \(\ce{HBr}\) | Hydrobromic acid |
| \(\ce{HI}\) | Hydroiodic acid |
| \(\ce{HNO}_{3}\) | Nitric acid |
| \(\ce{HClO}_{3}\) | Chloric acid |
| \(\ce{HClO}_{4}\) | Perchloric acid |
| \(\ce{H}_{2}\ce{SO}_{4}\) | Sulfuric acid |
In a strong acid, dissociation equilibrium lies more to the right side as dissociation is closer to 100%. For a weak acid, equilibrium lies more to the left side. Therefore, in a solution of a strong acid, dominant species are \(\ce{H}_{3}\ce{O}^{+}\) and \(\ce{A}^{-}\), while that in a weak acid is \(\ce{HA}\).
Strong Bases and Weak Bases
Strong bases show a high affinity for protons in an aqueous solution. For instance, \(\ce{NaOH}\), \(\ce{KOH}\) and hydroxides of main group one and two elements in the periodic table.
Weak bases show less affinity for protons in aqueous solutions. For instance, the solution of \(\ce{NH}_{3}\) gas in water.
In a solution of a strong base, the equilibrium lies more towards the right side. Therefore the dominant species in the solution are \(\ce{BH}^{+}\) and \(\ce{OH}^{-}\). In a solution of a weak base, the equilibrium lies more towards the left, which means the dominant species in the solution is \(\ce{B}\).
There is an inverse relationship between the strength of an acid and the strength of a base. The conjugate base of a strong acid is always a weak base, and the conjugate base of a weak acid is always a strong base. This relationship can be explained using the definition of a strong/weak acid and strong/weak base. The strong acid has the capacity to completely (nearly 100%) dissociate in water to donate protons, which means its conjugate base has less affinity to protons as the reaction equilibrium favours towards the right side (forward reaction predominates). A base showing less affinity for protons is a weak base. Similarly, in water, the weak acid partially dissociates to produce protons, which means its conjugate base displays a high affinity for the protons, as reaction equilibrium lies towards the left (reverse reaction predominates). Therefore, the stronger the acid, the weaker its conjugate base, and the weaker the acid, the stronger its conjugate base.
Acids and Bases Ionisation Constants
The strengths of acids and bases can be quantified using ionisation constants.
Dissociation of a weak acid in water can be written as: \[ \ce{HA}\left(aq\right)+\ce{H}_{2}\ce{O}\left(l\right)\rightleftharpoons\ce{H}_{3}\ce{O}^{+}\left(aq\right)+\ce{A}^{-}\left(aq\right) \]
Ionisation constant is a form of the equilibrium constant. Therefore, the ionisation constant of the above reaction can be written as follows: \[ K_{a}=\frac{\left[\ce{H}_{3}\ce{O}^{+}\right]\left[\ce{A}^{-}\right]}{\left[\ce{HA}\right]} \]
where
\(K_{a}\)= acid ionisation/dissociation constant
Water is not included in the ionisation constant expression as water is a pure liquid. The concentrations of \(\ce{H}_{3}\ce{O}^{+}\), \(\ce{A}^{-}\)and \(\ce{HA}\) are experimentally determined to calculate the ionisation constant. We can predict the strength of an acid using the acid ionisation constant. The strength of an acid is directly proportional to the acid ionisation constant. Strong acids have large \(K_{a}\)values (\(>1\)) as a forward reaction is favoured, leading to a larger concentration of \(\ce{H}_{3}\ce{O}^{+}\) and \(\ce{A}^{-}\) than \(\ce{HA}\). Weak acids possess \(K_{a}\) values less than \(1\), as ionisation (forward reaction) is not favoured.
1Electronegativity (EN) is the power in an atom in a molecule to attract electrons.