Acids, bases and salts
\[\require{mhchem}\]
Arrhenius acid-base theory
Arrhenius acid: Any substance that contains hydrogen and generates hydrogen ions in water. Examples - \(\ce{HCl}\) and \(\ce{HNO}_{3}\). \[ \ce{HNO}_{3}\left(l\right)\rightarrow\ce{H}^{+}\left(aq\right)+\ce{NO}_{3}^{-}\left(aq\right) \]
\[ \ce{HCl}\left(g\right)\rightarrow\ce{H}^{+}\left(aq\right)+\ce{Cl}^{-}\left(aq\right) \]
Arrhenius base: Any substance containing hydroxide and generating hydroxyl ions in water. Examples - \(\ce{NaOH}\) and \(\ce{KOH}\).
\[ \ce{NaOH}\left(s\right)\rightarrow\ce{Na}^{+}\left(aq\right)+\ce{OH}^{-}\left(aq\right) \]
\[ \ce{KOH}\left(s\right)\rightarrow\ce{K}^{+}\left(aq\right)+\ce{OH}^{-}\left(aq\right) \]
Limitations of the Arrhenius acid-base theory
Arrhenius’s acid base theory is limited to aqueous solutions. This theory can not explain why compounds without hydroxyl groups behave as bases, such as ammonia generating a basic aqueous solution.
Bronsted-Lowry acid-base theory
Due to the limitations in the Arrhenius acid-base theory, the Bronsted-Lowry Acid-Base Theory was introduced.
Bronsted-Lowry acid: any substance that can donate a hydrogen ion (or a proton) to another substance.
Bronsted-Lowry base: any substance that can accept a hydrogen ion (or a proton) from another substance1 \(=\) hydrogen ion/proton acceptor
Bronsted-Lowry acids can donate a different number of hydrogen ions. For instance, \(\ce{HCl}\) and \(\ce{HNO}_{3}\) can donate one hydrogen ion (monoprotic acids), \(\ce{H}_{2}\ce{SO}_{4}\) is capable of donating two hydrogen ions (diprotic acids), and \(\ce{H}_{3}\ce{PO}_{4}\) can donate three hydrogen ions (triprotic acids).
Not all hydrogens are acidic. Only the hydrogens bound to highly electronegative atoms such as oxygen and chlorine can be released as protons or hydrogen ions. Hydrogens in the examples above, \(\ce{HCl}\), \(\ce{HNO}_{3}\), \(\ce{H}_{2}\ce{SO}_{4}\), and \(\ce{H}_{3}\ce{PO}_{4}\) are all acidic as those hydrogens are bound to \(\ce{Cl}\), \(\ce{O}\). However, if you consider acetic acid \(\left(\ce{CH}_{3}\ce{COOH}\right)\), only one hydrogen out of four is acidic as one hydrogen connected to a highly electronegative atom, \(\ce{O}\) and the others are bound to \(\ce{C}\).
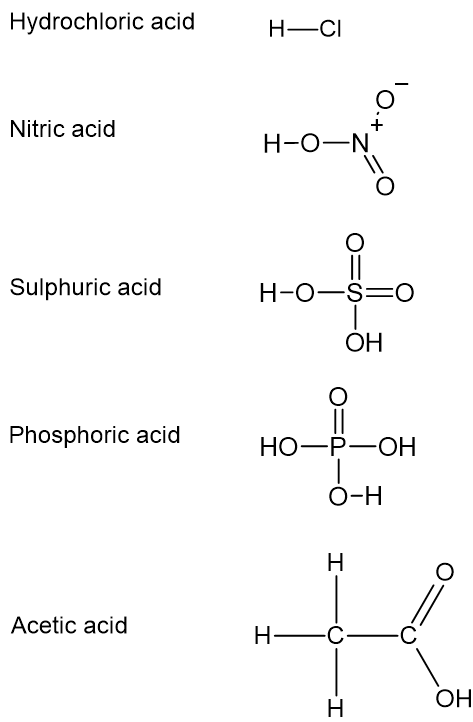
Structures of common acids
According to the Bronsted-Lowry acid-base theory, the presence of a species that can accept protons (a base) is vital for the existence of a Bronsted-Lowry acid. Proton donation and acceptance are complementary processes. For instance, consider the following reactions:
- When \(\ce{HCl}\) gas dissolves in water - \(\ce{HCl}\) donates the proton to the water (Bronsted-Lowry acid). The polar water molecules accept the proton (Bronsted-Lowry base) and become hydronium ions. The bond formed between the proton and the water molecule is a coordinate covalent bond as the oxygen atom supplies both electrons to the bond: \[ \ce{HCl}\left(g\right)+\ce{H}_{2}\ce{O}\left(l\right)\rightarrow\ce{H}_{3}\ce{O}^{+}\left(aq\right)+\ce{Cl}^{-}\left(aq\right) \] \(\ce{HCl}\) \(=\) Proton donor \(=\) Acid
\(\ce{H}_{2}\ce{O}\) \(=\) Proton acceptor \(=\) Base
- The reaction between \(\ce{NH}_{3}\) gas and \(\ce{HCl}\) gas - \(\ce{HCl}\) donates a proton to \(\ce{NH}_{3}\). Ammonia molecules accept the protons and become ammonium ions (\(\ce{NH}_{4}^{+}\)). The bond created between ammonia and the proton is a coordinate covalent bond as the nitrogen atom provides both electrons to the bond. \[ \ce{NH}_{3}\left(g\right)+\ce{HCl}\left(g\right)\rightarrow\ce{NH}_{4}^{+}\left(g\right)+\ce{Cl}^{-}\left(g\right) \] \(\ce{HCl}\) = Proton donor = Acid
\(\ce{NH}_{3}\) = Proton acceptor = Base
Bronsted-Lowry acids are denoted by \(\ce{HA}\). The \('H'\) represents the hydrogen atom, and the \('A'\) represents the rest of the acid.
Bronsted-Lowry bases are denoted by \(\ce{B}\colon\) as the main requirement of a Bronsted-Lowry base is to bear a lone pair of electrons.
The general equation of the Bronsted-Lowry acid-base reaction can be written as the following: \[ \ce{HA}+\ce{B}\colon\rightarrow\ce{A}^{-}+\left[\ce{B}\colon\ce{H}\right]^{+} \]
Conjugate Acid-Base Pairs
Many Bronsted-Lowry acid-base reactions are reversible.
Let’s consider the general equation for the Bronsted-Lowry acid-base reaction. This reaction consists of two acids and two bases: \[ \ce{HA}+\ce{B}\rightleftharpoons\ce{HB}^{+}+\ce{A}^{-} \]
In the forward reaction, \(\ce{HA}\) donates a proton to \(\ce{B}\). So, \(\ce{HA}\) functions as an acid and \(\ce{B}\) functions as a base: \[ \ce{HA}+\ce{B}\rightarrow\ce{HB}^{+}+\ce{A}^{-} \]
In the reverse reaction, \(\ce{HB}^{+}\)donates a proton to \(\ce{A}^{-}\). Therefore, \(\ce{HB}^{+}\) functions as an acid and \(\ce{A}^{-}\) functions as a base: \[ \ce{HB}^{+}+\ce{A}^{-}\rightarrow\ce{HA}+\ce{B} \]
\(\ce{HA}\), \(\ce{A}^{-}\) and \(\ce{B}\) , \(\ce{HB}^{+}\) are referred to as conjugate acid-base pairs. Conjugate acid-base pairs are always present on opposite sides of the chemical equation. For instance, \(\ce{HA}\) (conjugate acid) and \(\ce{A}^{-}\) (conjugate base).
In a conjugate acid-base pair, acid always has one more hydrogen atom than the base. For instance, \(\ce{CH}_{3}\ce{COOH}\) (acetic acid) dissociation in water. \[ \ce{CH}_{3}\ce{COOH}+\ce{H}_{2}\ce{O}\rightleftharpoons\ce{CH}_{3}\ce{COO}^{-}+\ce{H}_{3}\ce{O}^{+} \]
\(\ce{CH}_{3}\ce{COOH}/\ce{CH}_{3}\ce{COO}^{-}\) \(=\) conjugate acid-base pair. \(\ce{CH}_{3}\ce{COOH}\) has one hydrogen atom greater than the \(\ce{CH}_{3}\ce{COO}^{-}\) ion. So, \(\ce{CH}_{3}\ce{COOH}\) is the acid and \(\ce{CH}_{3}\ce{COO}^{-}\) is base in the conjugate acid-base pair.
1A hydrogen atom contains one electron and one proton. When a hydrogen atom becomes a hydrogen cation, it loses the electron. So the hydrogen ion only contains a proton. Therefore, we refer to a hydrogen ion as a proton. \(=\) hydrogen ion/proton donor.
