History of the periodic table
The periodic table is a single image that contains all the elements that we know exist in the universe. It classifies elements into categories and makes predictions about elements that have not yet been found by humans.
Alchemical table to periodic table
Before modern science, elements referred to earth, air, water, and fire. The periodic table grew from the alchemical table. The alchemical table was also a way of ordering elements and substances and their corresponding symbols. As more elements were discovered and a modern understanding of chemistry emerged, some things were removed from the table and others were added, beginning with phosphorus. Hennig Brand is the first person credited with discovering a modern element. Brand was an alchemist searching for the philosopher’s stone. He distilled many substances, including urine, and from human urine he made pure phosphorus.
Adding oxygen to the mix
Antoine Lavoisier is called the father of modern chemistry by many. Lavoisier discovered oxygen and hydrogen and correctly explained the role of oxygen in combustion – fire. Lavoisier also predicted the existence of silica. His work was ground-breaking and very important to modern chemistry. Many people contributed to the history of chemistry and the periodic table and discovered elements, building on the work of Lavoisier.
Atomic weight
Mendeleev was a Russian scientist who moved elements into the table structure we know today. HIs big breakthrough on the periodic table came in 1869 when he arranged the elements on the table by atomic weight, starting with the lightest elements. By ordering the elements by atomic weight, and into columns, he was able to correctly predict elements that had not yet been discovered. Mendeleev also knew that the proposed atomic weights of some elements were incorrect, and again correctly predicted what the actual weight is.
Periodic law
Grouping the elements by atomic weight also led to Mendeleev discovering the periodic law. This law states that by grouping elements by atomic weight, we see cyclical trends in their properties emerging in a predictable and stable way.
The Royal Society of Chemistry (RSC) have an interactive periodic table where you can learn the history of each element and who discovered it.
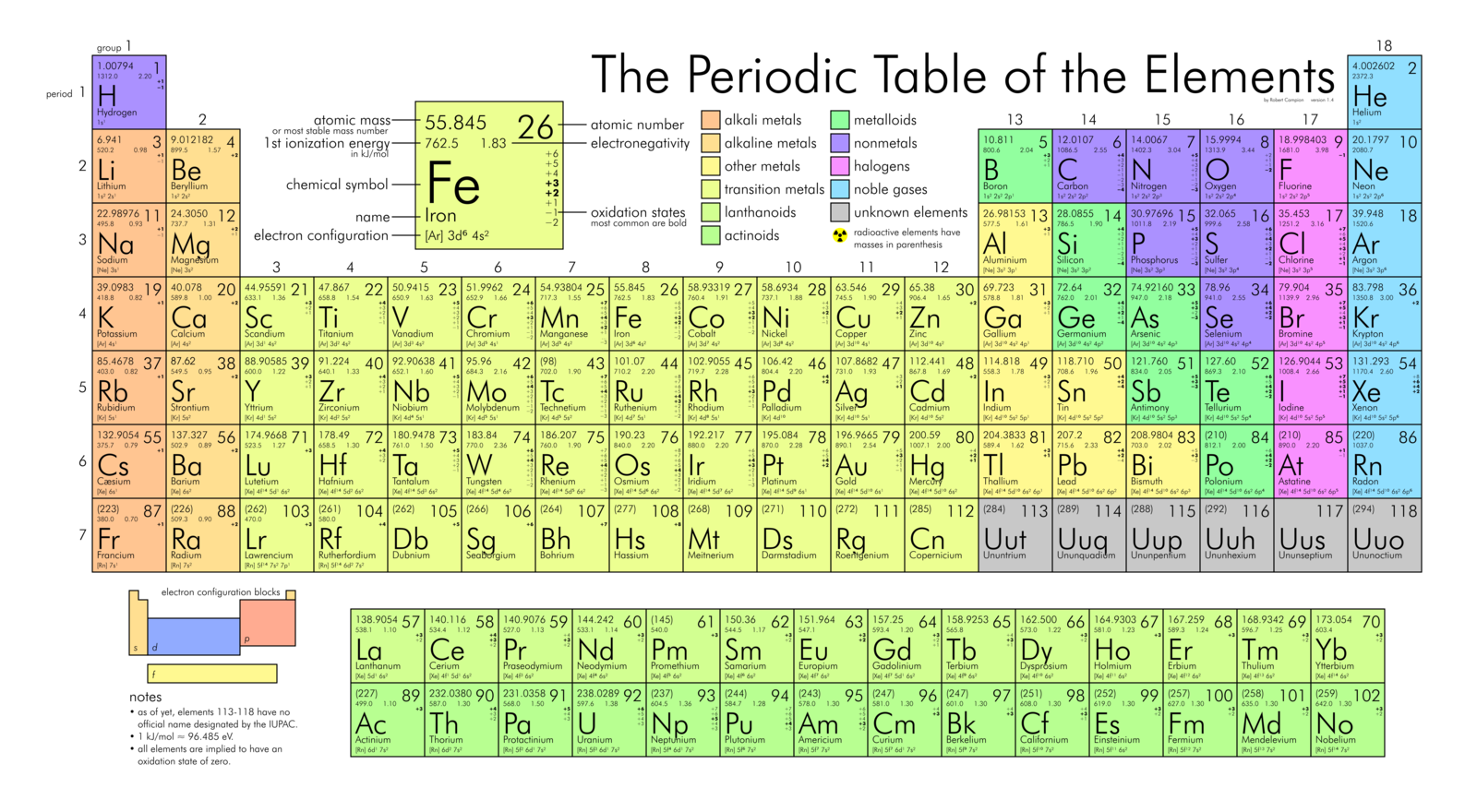
Image by 2012rc via Wikimedia Commons, licensed under CC BY 3.0
