States of matter
Explore states of matter further with this helpful resource.
In the study of chemistry, matter is the essential substance that makes up everything in our universe. Learn the basics of matter using this resource, including its states, properties and classification.
Matter exists in three physical states: solid, liquid and gas.
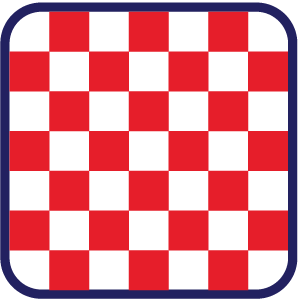 Solid: The particles in a solid are packed tightly with a regular pattern. They are held in place, giving solids a fixed shape and a fixed volume.
Solid: The particles in a solid are packed tightly with a regular pattern. They are held in place, giving solids a fixed shape and a fixed volume.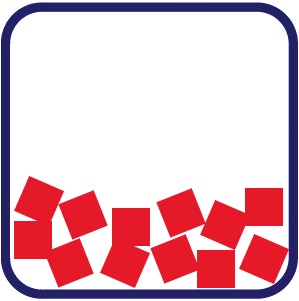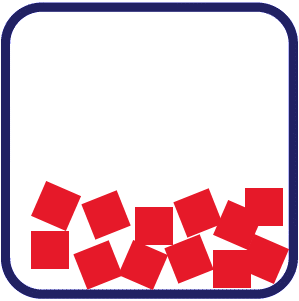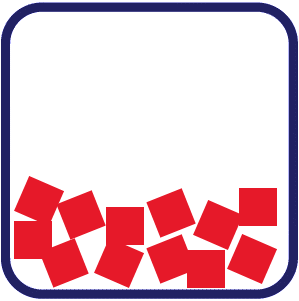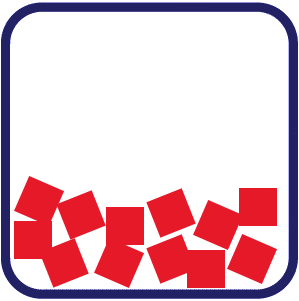 Liquid: In a liquid, particles are closely packed but lack a regular pattern, allowing them to slide past one another. This gives liquids an indefinite shape that conforms to their container, while maintaining a fixed volume.
Liquid: In a liquid, particles are closely packed but lack a regular pattern, allowing them to slide past one another. This gives liquids an indefinite shape that conforms to their container, while maintaining a fixed volume. Gas: The particles in a gas move quickly and are far apart from each other. This allows gas to take the shape of the container by filling the space. Gases have both an indefinite shape and volume.
Gas: The particles in a gas move quickly and are far apart from each other. This allows gas to take the shape of the container by filling the space. Gases have both an indefinite shape and volume.
States of matter images, by RMIT, licensed under CC BY-NC 4.0
In solids, the particles are tightly packed and arranged in a regular pattern. The particles are locked in place and have minimal movement.
In liquids, the particles are closely packed but lack a regular pattern. The particles are more free to move.
In gases, the particles move quickly and are far apart from each other. The particles are very free to move.
Properties of matter can be classified into two categories depending on whether it is determined by changing the chemical identity or composition of the substance.
If the property can be observed/measured without changing the chemical identity of the substance, it is a physical property. Examples of physical properties include:
If the property can only be observed or measured by altering the chemical identity of the substance, it is a chemical property. Examples of chemical properties include:
Matter can be classified in many ways. You can use the flowchart shown and answer a series of yes/no questions to classify matter.
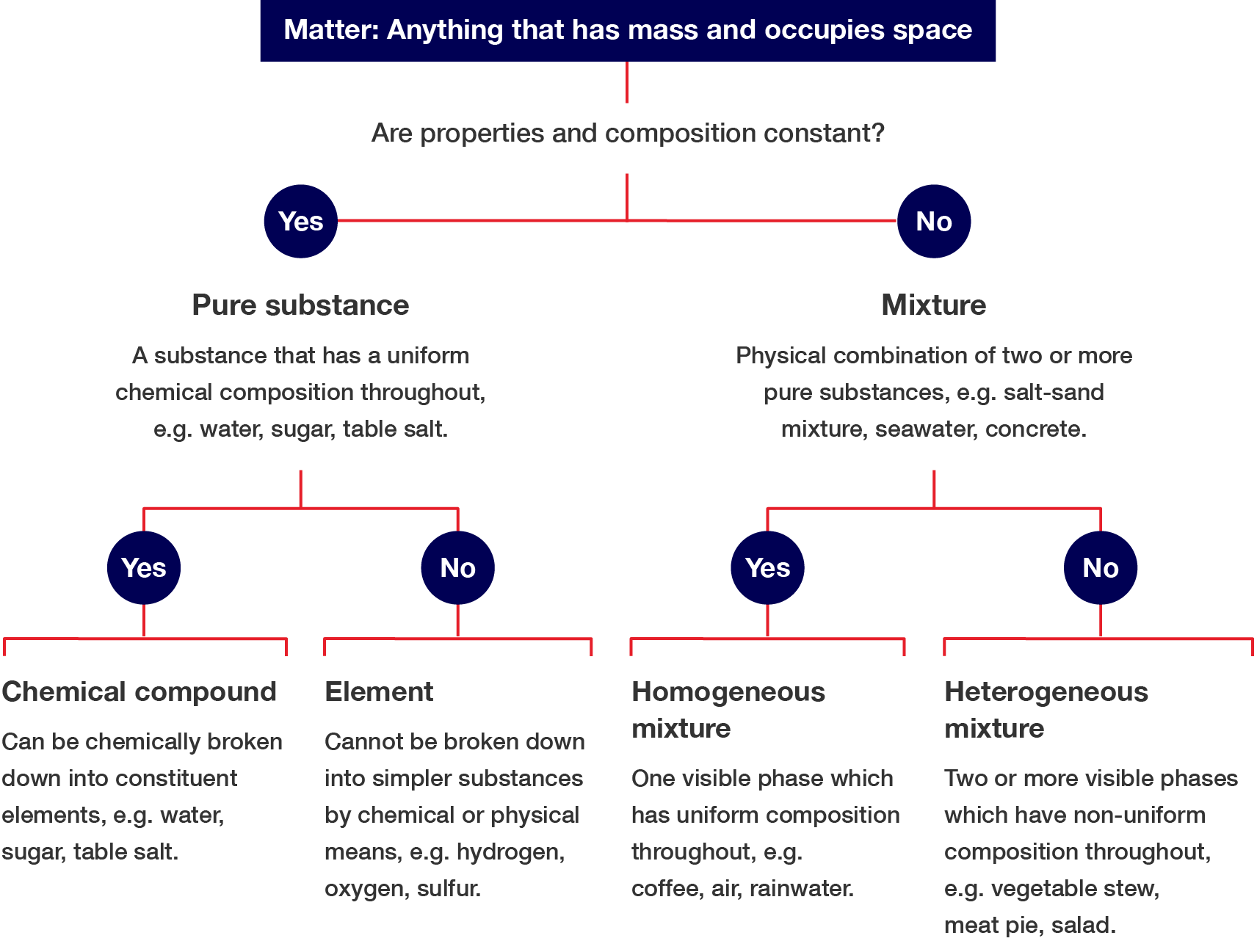
Are properties and composition constant?
Pure substance (a substance that has a uniform chemical composition throughout, e.g. water, sugar, table salt).
Can the pure substance be broken into simpler substances?
Mixture (A physical combination of two or more pure substances, e.g. salt–sand mixture, seawater, concrete.)
Does the mixture have a uniform composition throughout?
Test yourself on your understanding of the states, properties and classification of matter.