States of matter: Basics
Check out this PhET simulation on states of matter to explore how the particles behave during changes of state.
Matter is dynamic in nature. Explore how it undergoes physical and chemical changes, including transformations between different states such as melting, freezing, and evaporating.
A physical change is when the substance changes its physical appearance or state without affecting its chemical identity. Examples of physical changes include:
When a substance converts from one physical state to another, this is known as a change of state. Let's look at changes of state using water as an example.
Water is one of the few substances that can exist in all three states: solid ice, liquid water, and gaseous steam or vapour. The process of state changes have different names depending on which states are involved.
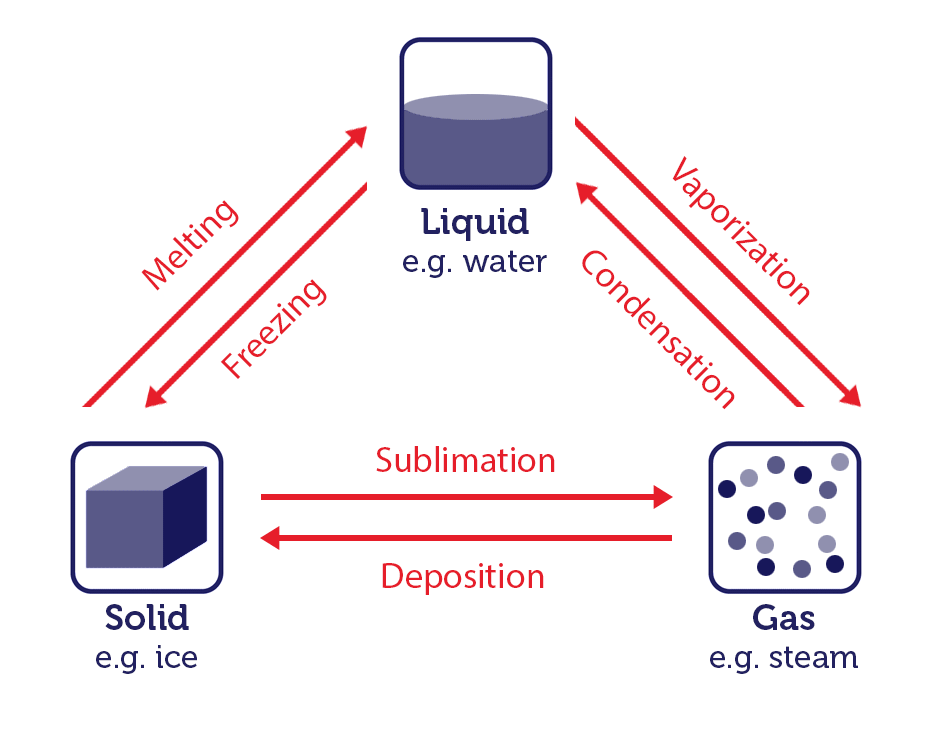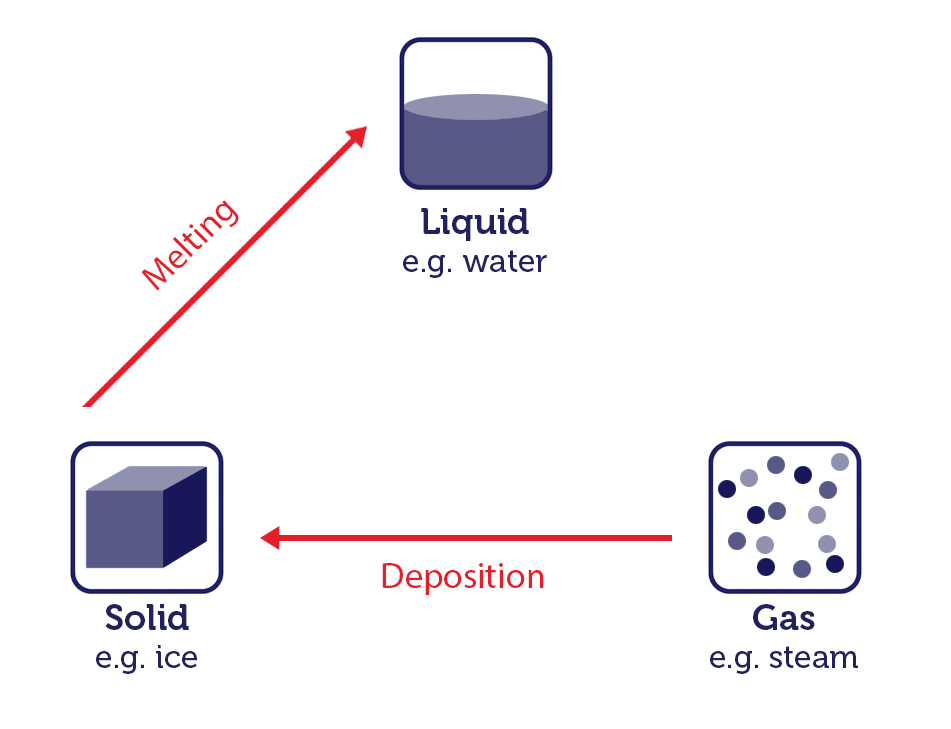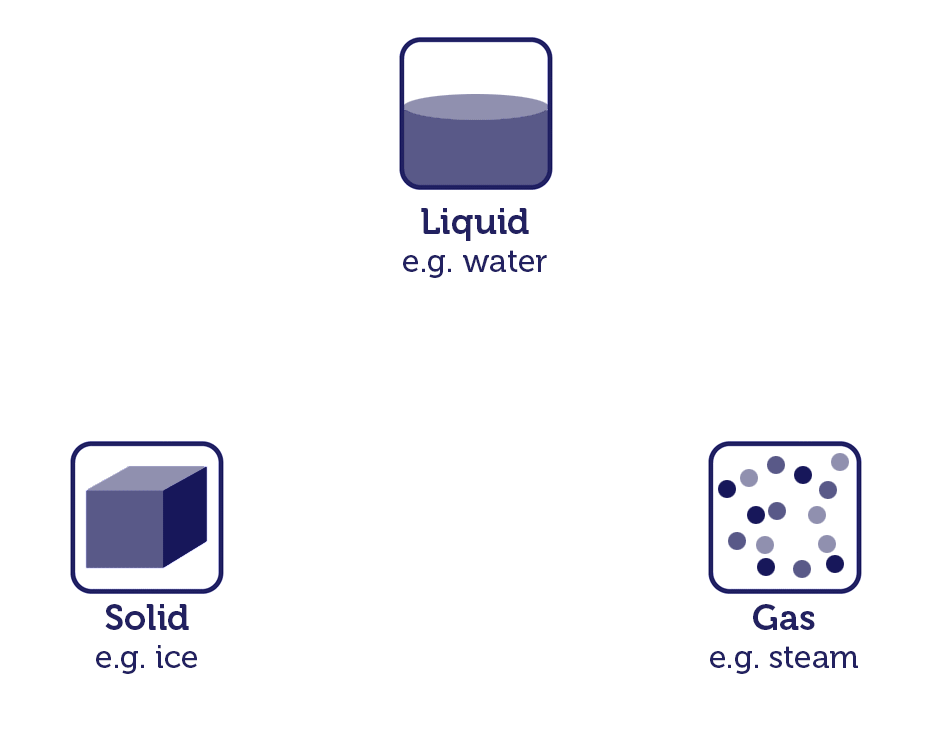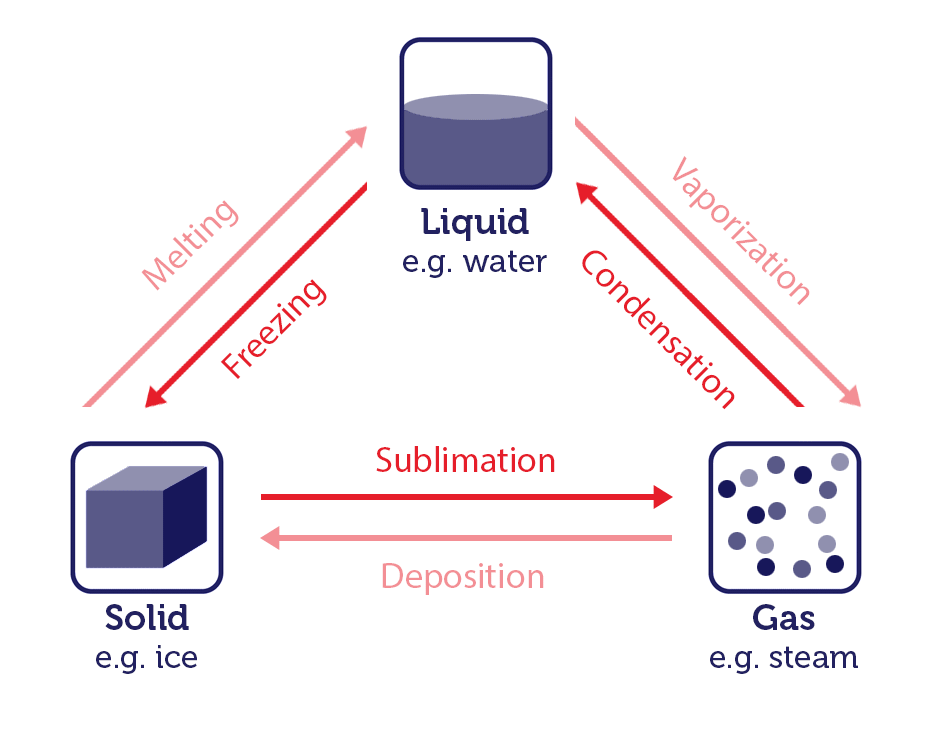
Changing states of matter image, by RMIT, licensed under CC BY-NC 4.0
A chemical change is when a substance changes its chemical identity or composition. It involves the formation of new substances that have different properties compared to the original substance. Examples of chemical changes include:
Test yourself on your understanding of physical and chemical changes.