Atoms: The building blocks of everything
Use this helpful resource to learn more about atoms, molecules and compounds.
Atoms are the basic building blocks of elements. They are too small to be observed by the naked eye. Let's explore atoms, and molecules and compounds.
Elements are the building blocks of all matter and composed of one type of atom, such as \(\ce{He}\), \(\ce{H}\), \(\ce{C}\), \(\ce{Mg}\), and \(\ce{Fe}\). Some elements exist as molecules, such as \(\ce{S}_8\), \(\ce{O}_2\), and \(\ce{Cl}_2\).
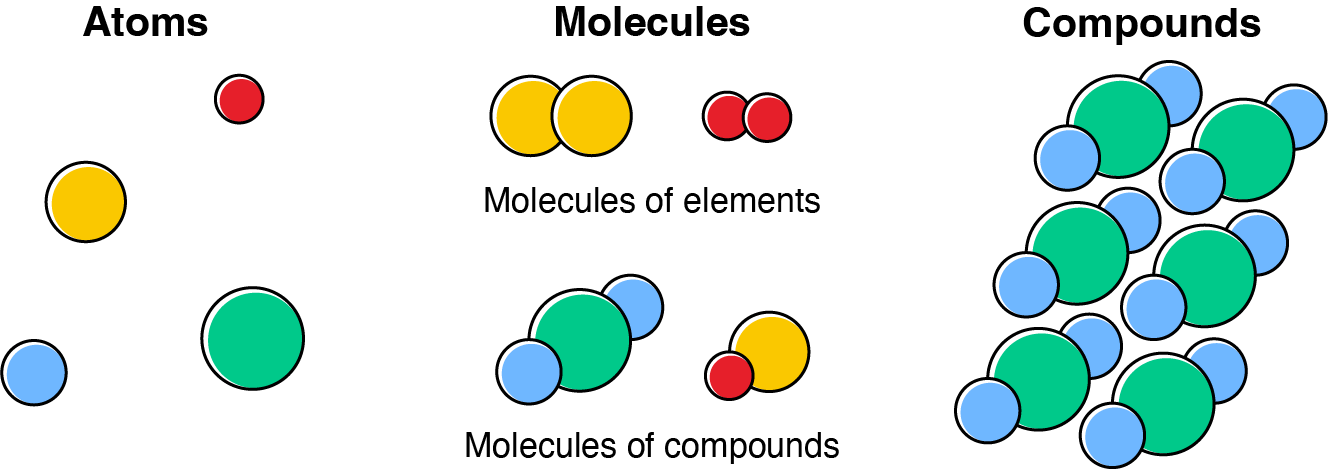
A molecule is a group of two or more atoms that have combined chemically and function as a unit. The simplest molecule that can exist is a diatomic molecule that contains two atoms. Two examples are \(\ce{O}_2\) and \(\ce{H}_2\). A compound contains different types of atoms in fixed proportions.
Both compounds and molecules are uncharged (neutral) species. Some examples are shown in the table.
| Molecules | Compounds |
|---|---|
| \(\ce{H2}\) | \(\ce{H2O}\) |
| \(\ce{O3}\) | \(\ce{CO2}\) |
| \(\ce{S8}\) | \(\ce{C2H5OH}\) |
| \(\ce{CO2}\) | \(\ce{CH3COOH}\) |
| \(\ce{H2O}\) | \(\ce{FeSO4}\) |
Each element has a unique name and chemical symbol. All of the discovered elements are organised in the modern periodic table. Some of these are shown in the table.
| Element | Chemical symbol |
|---|---|
| Hydrogen | \(\ce{H}\) |
| Carbon | \(\ce{C}\) |
| Calcium | \(\ce{Ca}\) |
| Silver | \(\ce{Ag}\) |
| Copper | \(\ce{Cu}\) |
Chemical formulas represent the chemical composition of molecules and compounds. They consist of the chemical symbols for the elements present in the compound and numerical subscript which shows the number of atoms of each element involved in the formation of the compound.
Consider the chemical formula for the compound sucrose, \(\ce{C12H22O11}\).
Now, consider the chemical formula for the compound calcium phosphate: \(\ce{Ca3(PO4)2}\).
Test yourself on your understanding of atoms, molecules and compounds.
Images on this page by RMIT, licensed under CC BY-NC 4.0