Definitions and theories of acids and bases
Explore the definitions and theories of acids and bases in more detail using this helpful resource.
Acids and bases are vital chemical compounds with significant roles in both science and daily life. Acids, with their sour taste and reactivity, are found in citrus fruits and vinegar, while bases, known for their bitter taste and slipperiness, appear in cleaning products and baking soda. When acids and bases react, they form salts, compounds that are essential in everything from seasoning food to preserving life through electrolytes. Understanding these compounds is key to grasping many chemical reactions and processes.
Acids and bases can be easily classified based on their useful properties, but how are they defined from a chemical perspective?
Arrhenius' theory of acids and bases defines acids and bases in terms of their behaviour in water.
Arrhenius's theory is limited to aqueous solutions, since it specifically defines acids and bases based on their behaviour in water. This theory cannot explain why compounds without hydroxyl groups behave as bases, such as ammonia generating a basic aqueous solution.
Due to the limitations of Arrhenius' theory of acids and bases, the Brønsted–Lowry acid–base theory was introduced.
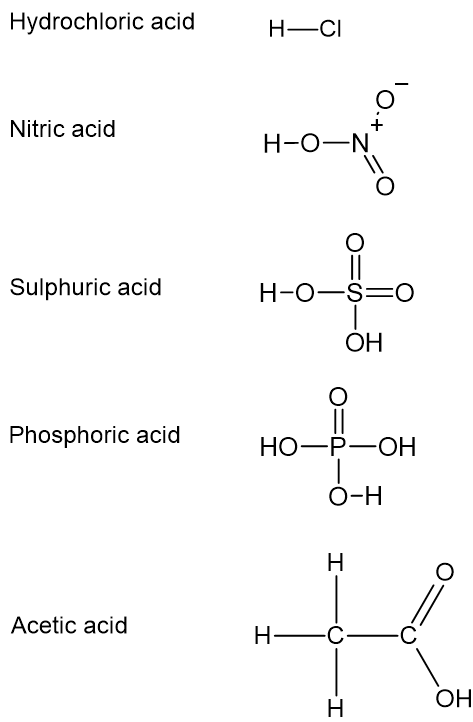
Brønsted–Lowry acids such as \(\ce{HCl}\) and \(\ce{HNO3}\) can donate one hydrogen ion, so they are called monoprotic acids. Some Brønsted–Lowry acids can donate multiple hydrogen ions. For example, \(\ce{H2SO4}\) is capable of donating two hydrogen ions (diprotic acid), and \(\ce{H3PO4}\) can donate three hydrogen ions (triprotic acid).
Not all hydrogens are acidic. Only the hydrogens bound to highly electronegative atoms such as oxygen and chlorine can be released as protons or hydrogen ions. Hydrogens in \(\ce{HCl}\), \(\ce{HNO3}\), \(\ce{H2SO4}\), and \(\ce{H3PO4}\) are all acidic as the hydrogens are bound to electronegative \(\ce{Cl}\) and \(\ce{O}\). However, if you consider acetic acid, \(\ce{CH3COOH}\), only one of the four hydrogens is acidic. This is the hydrogen atom connected to the highly electronegative atom, \(\ce{O}\). The others are bound to \(\ce{C}\).
Examine the structures below and determine which of the hydrogens in each are acidic.
The presence of a species that can accept protons (a base) is vital for the existence of a Brønsted–Lowry acid, and vice versa. Proton donation and acceptance are complementary processes. The general equation of the Brønsted–Lowry acid–base reaction is:
\[ \ce{HA}+\ce{B}\colon\rightarrow\ce{A}^{-}+\left[\ce{B}\colon\ce{H}\right]^{+} \]
Let's look at two examples written in this format.
Many Brønsted–Lowry acid–base reactions are reversible. After donating protons, acids are able to act as bases and accept protons. In the same way, after accepting a proton, bases can act as acids and donate the proton.
Let's consider the general equation for the Brønsted-Lowry acid-base reaction. This reaction involves two acids and two bases:
\[ \ce{HA}+\ce{B}\rightleftharpoons\ce{HB}^{+}+\ce{A}^{-} \]
\(\ce{HA}\)/\(\ce{A}^{-}\) and \(\ce{B}\)/\(\ce{HB}^{+}\) are referred to as conjugate acid–base pairs. Conjugate acid–base pairs are always present on opposite sides of the chemical equation. For instance, \(\ce{HA}\) (acid) and \(\ce{A}^{-}\) (conjugate base).
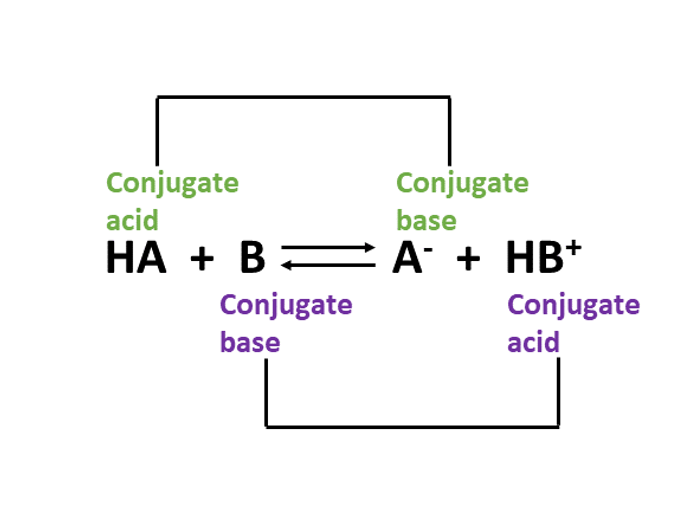
In a conjugate acid–base pair, the acid always has one more hydrogen atom than the base. For example, the reaction for the dissociation of \(\ce{CH}_{3}\ce{COOH}\) (acetic acid) in water is:
\[ \ce{CH3COOH}+\ce{H2O}\rightleftharpoons\ce{CH3COO}^{-}+\ce{H3O}^{+} \]
\(\ce{CH3COOH}/\ce{CH3COO}^{-}\) is a conjugate acid–base pair. \(\ce{CH3COOH}\) has one hydrogen atom more than the \(\ce{CH3COO}^{-}\) ion. So, \(\ce{CH3COOH}\) is the acid and \(\ce{CH3COO}^{-}\) is base in the conjugate acid-base pair.
Identify the following substances as a Brønsted-Lowry acid or base:
Step 1: Recall the definitions of a Brønsted–Lowry acid and a Brønsted–Lowry base.
A Brønsted–Lowry acid is any substance that can donate a hydrogen ion (or a proton) to another substance; they typically have one or more hydrogen atoms attached to a highly electronegative atom. A Brønsted–Lowry base is any substance that can accept a hydrogen ion (or a proton) from another substance; they typically contain a lone pair.
Step 2: Identify whether the substance can donate hydrogen ions or accept hydrogen ions.
Write the formula for the:
Step 1: Write the equations for the dissociation of each acid or base.
Step 2: In a conjugate acid–base pair, the acid always has one more hydrogen atom than the base. Therefore, the conjugate base is the species with one less hydrogen atom, and the conjugate acid is the species with one more hydrogen atom.
\(\ce{H3PO4}\) is an acid that donates a hydrogen ion to become \(\ce{H2PO}_{4}^{-}\). Therefore, the conjugate base of \(\ce{H3PO4}\) is the \(\ce{H2PO}_{4}^{-}\) ion.
As a base, \(\ce{NO}_{3}^{-}\) accepts a hydrogen ion to become \(\ce{HNO3}\). Therefore, the conjugate acid of \(\ce{NO}_{3}^{-}\) is the \(\ce{HNO3}\).
Images on this page by RMIT, licensed under CC BY-NC 4.0