Covalent bonds and polarity
Use this resource to learn more about covalent bonding and polarity.
Covalent bonding is responsible for creating many substances we encounter in our daily lives. Water (\(\ce{H2O}\)) is essential for life, carbon dioxide (\(\ce{CO2}\)) is a key component of the Earth's atmosphere, and methane (\(\ce{CH4}\)) is a common fuel source. Use this resource to learn more about how covalent bonds form.
Covalent bonding primarily occurs between non-metal atoms or elements located in the \(p\)-block. Whereas ionic bonding involves the transfer of valence electrons, covalent bonding involves the sharing of valence shell electrons to achieve a complete outer shell. Atoms held together by covalent bonds are called molecular compounds. The figure below shows the formation of three covalent molecular compounds: \(\ce{H}_{2}\), \(\ce{F}_{2}\) and \(\ce{H}_{2}\ce{O}\).
Hydrogen molecule (H2): Fluorine molecule (F2): Water molecule (H2O):
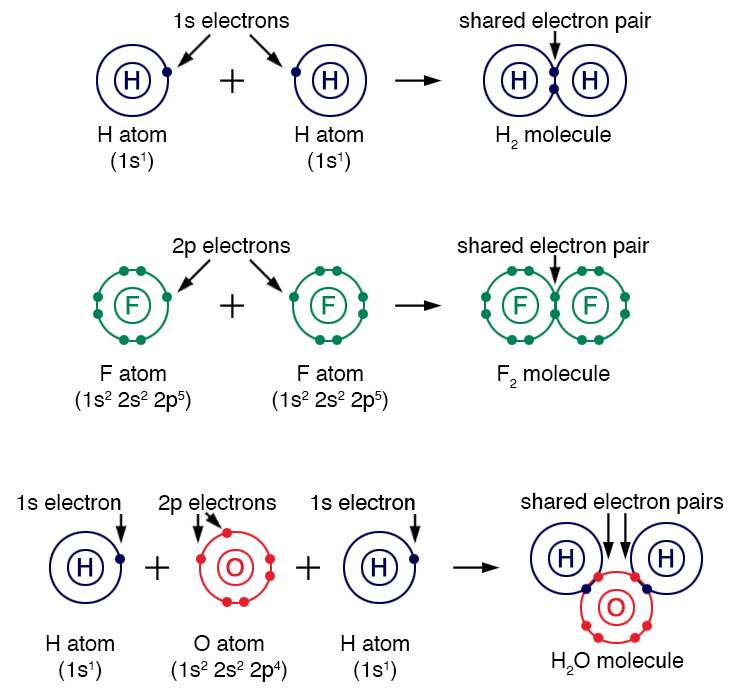
Two hydrogen atoms (H), each with 1s electron, combine to form an H2 molecule with a shared electron pair.
Two fluorine atoms (F), each with 2p electrons, combine to form an F2 molecule with a shared electron pair.
Two hydrogen atoms and one oxygen atom combine. The oxygen atom (with 2p electrons) shares electrons with two hydrogen atoms to form an H2O molecule, showing shared electron pairs.
Generally, the number of covalent bonds an atom forms depends on how many electrons an atom requires to achieve an octet. For instance, \(\ce{C}\) forms four covalent bonds as \(\ce{C}\) \(\left(1s^{2}\,2s^{2}\,2p^{2}\right)\) needs four more electrons to acquire an octet in their valence shell. A few more examples are listed in the table.
| Element | Electron configuration | Electrons required to fill the octet | Number of covalent bonds |
|---|---|---|---|
| \(\ce{N}\) | \(1s^{2}\,2s^{2}\,2p^{3}\) | \(3\) | \(3\) |
| \(\ce{O}\) | \(1s^{2}\,2s^{2}\,2p^{4}\) | \(2\) | \(2\) |
| \(\ce{F}\) | \(1s^{2}\,2s^{2}\,2p^{5}\) | \(1\) | \(1\) |
| \(\ce{H}\) | \(1s^{1}\) | \(1\) | \(1\) |
However, the number of covalent bonds formed by an atom is not always guided by the octet rule. Let's take \(\ce{S}\) as an example. \(\ce{S}\) can form four or six covalent bonds, such as \(\ce{SF}_{4}\) and \(\ce{SF}_{6}\). Some other exceptions to the octet rule are \(\ce{BF}_{3}\), \(\ce{PCl}_{5}\) and some of the \(p\) block elements in period \(4\), as they have empty \(d\) orbitals.
When two electrons are shared between two atoms, a single bond forms.
Multiple covalent bonds are formed between two atoms when they share more than one pair of electrons to acquire valence shell octet. For instance, \(\ce{CO}_{2}\): \(\ce{C}\) forms two covalent bonds (two pairs of electrons or four electrons) with each oxygen atom involved. These are called double bonds.
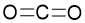
In the same way, when six electrons are shared between two atoms, a triple bond forms. Ethyne is an example of a molecular compound that forms a triple bond. One carbon atom shares six electrons with another carbon atom to complete the octet.

Typically, each atom contributes one or more electrons to the covalent bond. When the electrons shared between two atoms are donated by only one atom, the resulting bond is known as a coordinate covalent bond. In a coordinate covalent bond, an atom with a filled orbital shares its two electrons with an empty orbital of another atom.
Coordinate covalent bonds:
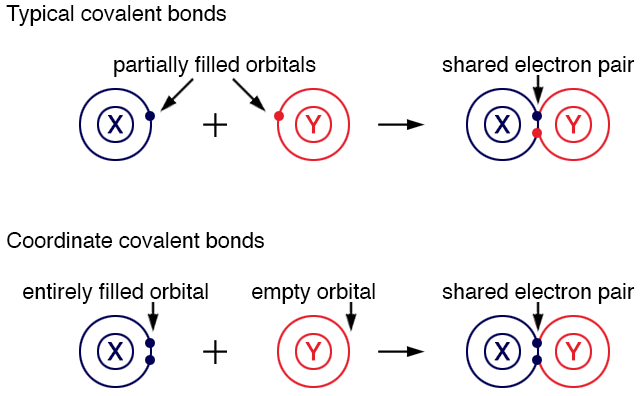
Shows two atoms, X and Y, with partially filled orbitals. They combine to form a shared electron pair.
Illustrates atom X with an entirely filled orbital and atom Y with an empty orbital. They combine to form a shared electron pair.
Examples of molecular compounds with coordinate covalent bonds are carbon monoxide (\(\ce{CO}\)) and ozone (\(\ce{O3}\)).
The three-dimensional arrangement of a molecular compound gives it a molecular shape. Shape is primarily determined by valence shell electron repulsion (VSEPR) theory. Being able to draw Lewis structures also helps with determining molecular shape.
VSEPR theory states that the shape of a molecule is determined by the number of electron clouds around the central atom. Non-bonding (lone pair) and bonding valence electrons are considered electron clouds. Due to the repulsion forces between electron clouds, they attempt to stay as far as possible.
For instance, a carbon dioxide molecule has two electron clouds (two \(\ce{C}-\ce{O}\) bonds) around the central atom \(\ce{C}\). As a result of repulsion forces between these two electron clouds, the molecule is arranged into linear geometry with a bond angle of \(180^{\circ}\). Multiple bonds, such as double and triple bonds, are considered one electron cloud.
Depending on the number of electron clouds around a central atom, the number of bonds formed and the number of lone pairs, the molecular shape will vary. The different molecular shapes are summarised in the table below.
| Number of bonds | Number of lone pairs | Total number of electron clouds | Shape | Example |
|---|---|---|---|---|
| 2 | 0 | 2 | Linear | 
|
| 2 | 1 | 3 | Angular/bent | 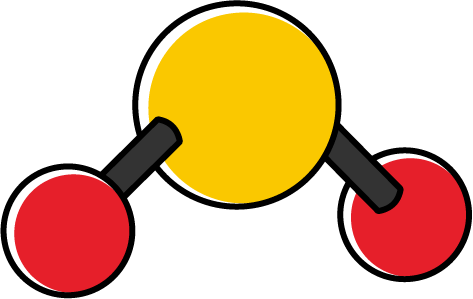
|
| 2 | 2 | 4 | Angular/bent | 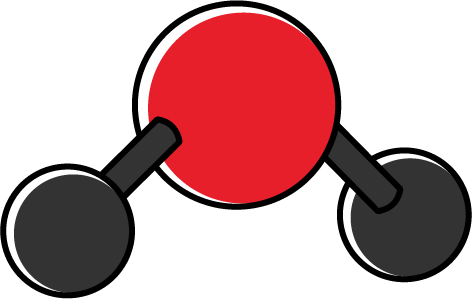
|
| 3 | 0 | 3 | Trigonal planar | 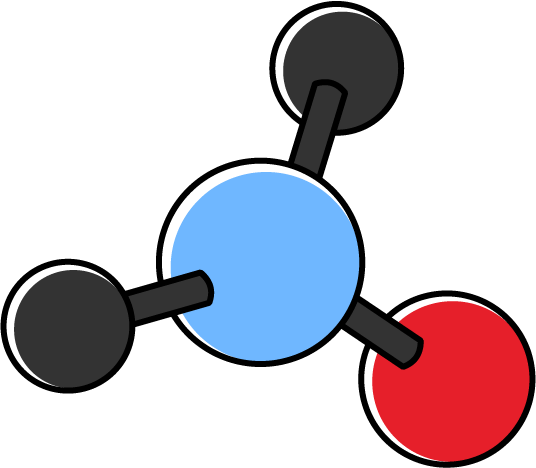
|
| 3 | 1 | 4 | Pyramidal | 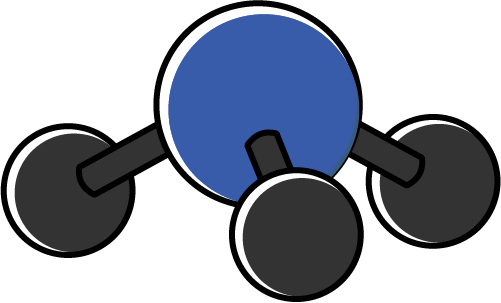
|
| 4 | 0 | 4 | Tetrahedal | 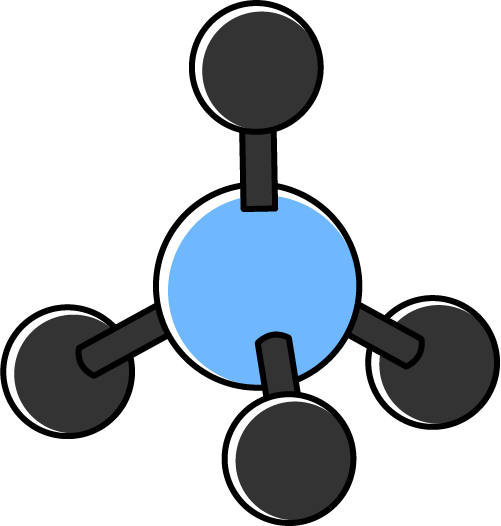
|
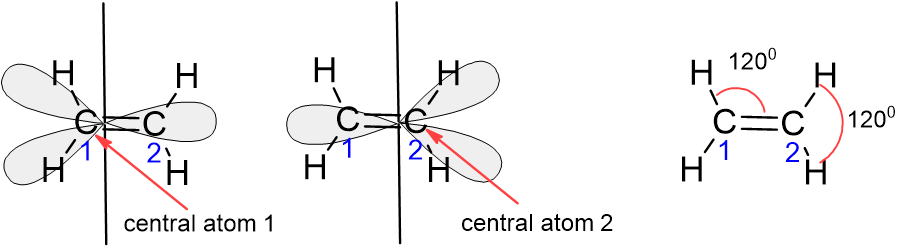
When more than one central atom is present in the molecule, the molecular geometry of each central atom is predicted and the results are combined. For example, \(\ce{C}_{2}\ce{H}_{4}\) has two central \(\ce{C}\) atoms. Each \(\ce{C}\) atom is attached to three electron clouds. This means each \(\ce{C}\) atom has a trigonal planar geometry. In this case, the whole molecule is also planar, as the bond angles \(\ce{H}-\ce{C}-\ce{H}\) and \(\ce{H}-\ce{C}-\ce{C}\) are \(120^{\circ}\) each.
In chemistry, polarity refers to the distribution of electric charge around atoms or molecules. The concept of polarity is crucial in understanding how covalent bonds and entire molecules interact with each other, influencing their physical and chemical properties.
In the context of covalent bonds, polarity arises when there is an unequal sharing of electrons between atoms due to differences in their electronegativities. This results in one end of the bond having a partial negative charge (designated \(\delta-\)) and the other a partial positive charge (designated \(\delta+\)). The partially negative end is electron-rich, while the partially positive end is electron-poor. This creates something called a dipole moment.
A covalent bond in which this occurs is called a polar covalent bond. One example is \(\ce{HF}\). The electronegativity (EN) of the \(\ce{H}\) atom is \(2.20\). The EN of the \(\ce{F}\) atom is \(3.98\). The difference in EN is \(3.98-2.20=1.78\). Valence electrons shared by \(\ce{H}\) and \(\ce{F}\) are more attracted to the electronegative \(\ce{F}\) atom. The \(\ce{F}\) atom therefore has a partial negative charge and is labelled \(\delta-\), and the \(\ce{H}\) atom has a partial positive charge and is labelled \(\delta+\).
A bond in which electrons are shared equally is simply called a non-polar covalent bond. An example is the bond between the two \(\ce{H}\) atoms that make \(\ce{H2}\). The difference in EN is \(0\).
The difference between the EN of participating atoms in a bond also indicates whether the bond is ionic, non-polar covalent or polar covalent. Compared to ionic bonds, the difference between the EN of atoms participating in covalent bonding is much smaller.
| Electronegativity difference | Type of bond | Examples |
|---|---|---|
| \(\geqslant2.0\) | Ionic | \(\ce{NaCl}\), \(\ce{CsF}\) |
| \(0.5-1.9\) | Polar covalent | \(\ce{HCl}\), \(\ce{H}_{2}\ce{O}\) |
| \(0-0.4\) | Covalent | \(\ce{CH}_{4}\), \(\ce{O}_{2}\) |
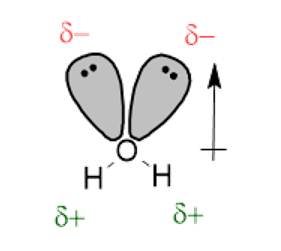
When discussing molecules, polarity depends on the polarity of the individual bonds, the contribution from lone pair electrons, and the molecular shape. In a polar molecule, electrons are more strongly attracted to one end of the molecule. For example, in the \(\ce{H2O}\) molecule, due to the high EN of the \(\ce{O}\) atom, electrons are strongly attracted to the \(\ce{O}\) atom. This creates a net polarity point between two \(\ce{O}-\ce{H}\) bonds. The polarity of the molecule is indicated using a crossed arrow that points in the direction of electron attraction.
In some cases, although the individual covalent bonds are polar, overall the molecule becomes non-polar due to the shape of the molecule. For instance, a carbon dioxide molecule has polar \(\ce{C}-\ce{O}\) bonds. However, due to the linear symmetrical shape of the molecule, polar bonds (dipoles) cancel each other, creating a zero net polarity.
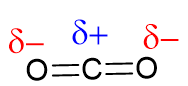
To name molecular compounds, you can follow the steps below. Let's use \(\ce{CCl4}\) as an example.
Some other examples are "carbon monoxide" for \(\ce{CO}\), "carbon dioxide" for \(\ce{CO2}\), "boron trichloride" for \(\ce{BCl3}\) and "dinitrogen pentoxide" for \(\ce{N2O5}\).
Images on this page by RMIT, licensed under CC BY-NC 4.0