Electrical principles - Principles
Atomic structure
Everything in the universe is made up of matter. Matter can exist as liquid, solid or gas.
Understanding atomic structure (the structure of matter) can help you to understand electricity.
Atomic theory
Matter can be broken down into small particles, called atoms. It takes billions of atoms to make up something as small as a drop of water. To understand electricity, we need to look at the inside of the atom.
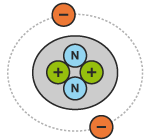
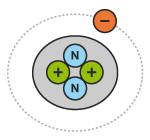
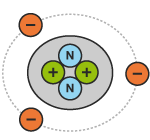
An atom is made up of protons, neutrons and electrons (see diagram below). Protons have a positive charge (+). Electrons have a negative charge (-). Neutrons have no charge (neutral).
Protons and neutrons are gathered together in the centre of the atom, or nucleus. Electrons spin around the nucleus in an orbit, like the planets spin around the sun.
If an atom has the same number of electrons and protons, it will be balanced and have a neutral charge (no charge).
If an atom has more electrons than protons, it will be unbalanced and be negatively charged. The following diagrams show an atom of helium in three different states of charge:
Electron flow
If an electromotive force (EMF) is applied across a conductor, or conductive circuit, eg a length of wire, electrons from each atom can be forced out of their orbit. These electrons are then capable of moving to another atom.
Electrons flow from the negative terminal of a power supply towards the positive terminal of the power supply through an electrical circuit. This is because the atoms on the negative side have an excess of electrons, so they are negatively charged. The atoms on the positive side have a deficiency of electrons, so they are positively charged. When connected together via a conductive circuit, or wire, there is a path for electrons to follow. The movement of the electrical charge around a circuit is the basis of electricity.
The above diagram shows the movement of electrons from atom to atom in a conductor.
As the electrons move through the conductor, they attach themselves to an atom and displace other electrons. The displaced electrons then move on to another atom, forcing other electrons to be displaced. The movement of electrons from one atom to the next is called 'electron flow'.
Electron flow can occur through solids, liquids and gases.
Current flow
In the past, current was assumed to flow through a conductor from positive to negative. Many rules were formulated based on this assumption and are still used today. Conventional current is still considered to flow from positive to negative, even though it is known that electrons move in the opposite direction.
This theory is called 'conventional current flow theory'.
Note: The diagrams throughout this resource use current flow theory. They represent current flow with arrows from positive to negative.



